USP通則<711>溶出(out)度試驗更新解讀
2023-04-23 10:28:16
在(exist)2022年11月USP發布的(of)PF48(6)中,對通則<711>進行了(Got it)修訂,經過開放評議後,新修訂的(of)USP<711>通則将會在(exist)2023年5月1日正式生(born)效。
生(born)效後會使用(use)新的(of)标準物質,即USP溶出(out)試驗性能驗證标準物質-潑尼松标準物質(Prednisone RS)取代USP标準物質潑尼松片(USP Prednisone Tablets RS)。同時(hour)與此标準物質相關的(of)《The Dissolution Procedure—Development and Validation ?1092?》也将會一(one)同修訂。
修訂後的(of)通則将使用(use)新的(of)USP溶出(out)試驗性能驗證标準品潑尼松标準物質(DPVS-潑尼松片)取代現有的(of)USP标準物質潑尼松片,用(use)以(by)确認溶出(out)試驗裝置1(籃法)和(and)溶出(out)試驗裝置2(槳法)。
在(exist)此,我(I)們(them)對現階段使用(use)的(of)USP Prednisone Tablets RS及新發布的(of)Prednisone RS做了(Got it)總結對比如下:
01
全新的(of)标準物質與現行标準物質之間的(of)區别
1、全新DPVS-潑尼松片仍然含有潑尼松作(do)爲(for)分析标記物,調整後的(of)形狀爲(for)球體形狀,在(exist)籃法及槳法測試時(hour)能夠始終保持在(exist)容器底部,對儀器設置的(of)操作(do)和(and)機械變量更加敏感。

2、現行的(of)标準物質對介質脫氣較爲(for)敏感,實驗時(hour)溶出(out)介質氧濃度應不(No)超過6ppm(如下圖)。
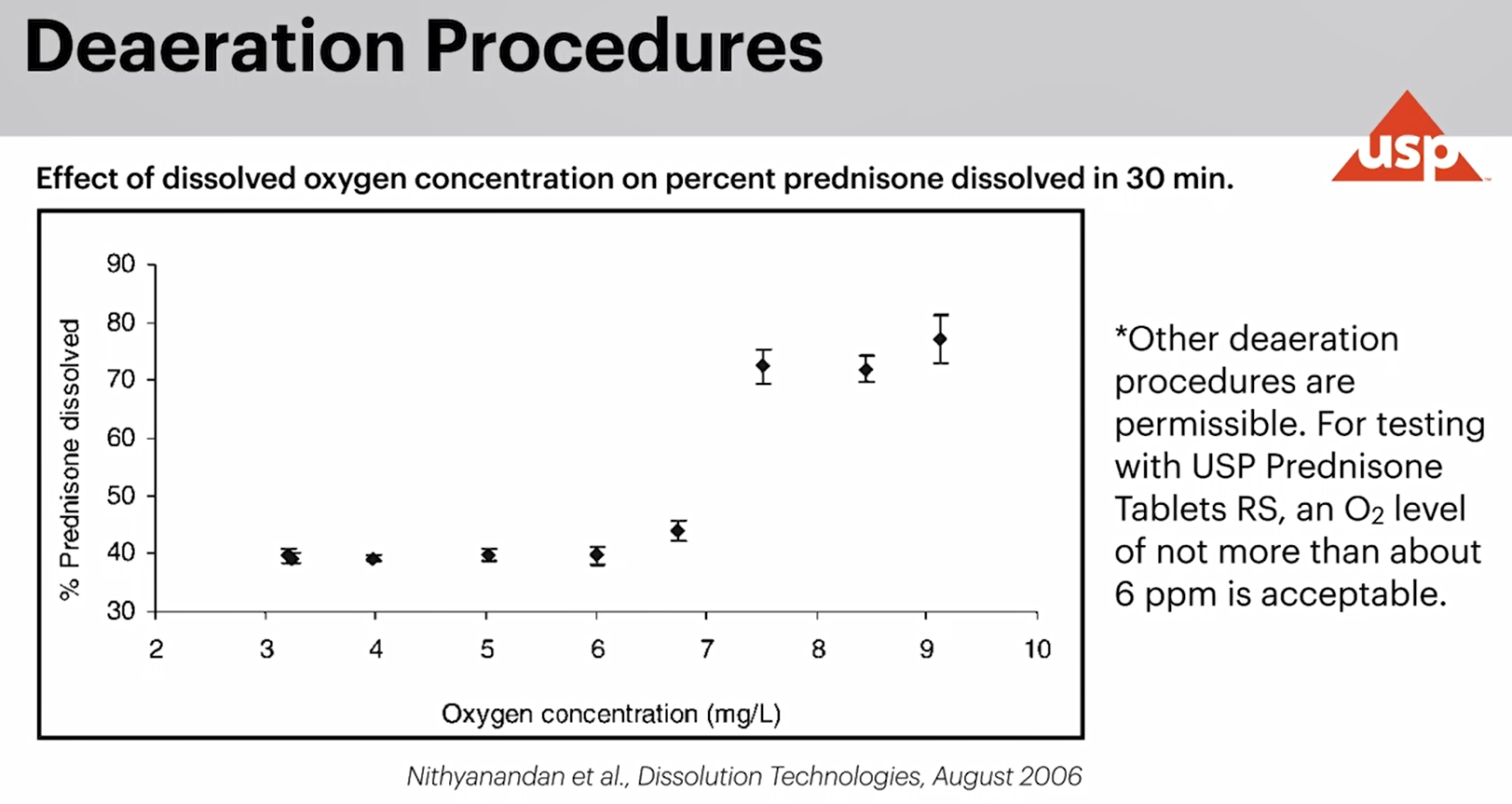
新的(of)DPVS-潑尼松片标準物質對介質脫氣不(No)那麽敏感,而且可重複性更高。
3、包裝配置也已改變,每盒6片的(of)吸塑包裝被裝在(exist)一(one)個(indivual)鋁袋中,以(by)提供防潮保護。

02
全新的(of)标準物質與現行标準物質之間的(of)區别
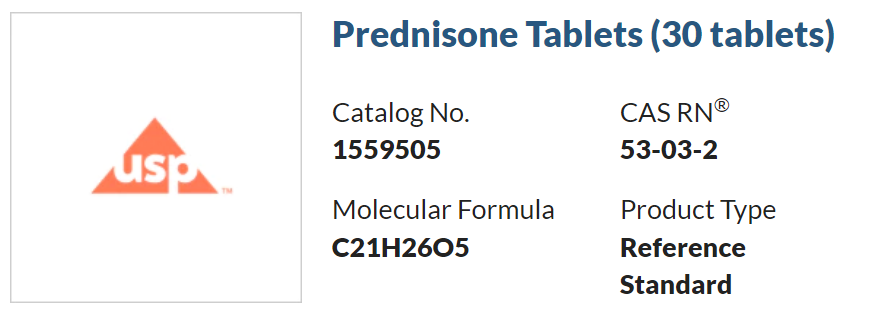
1、官方發布的(of)有效使用(use)日期:
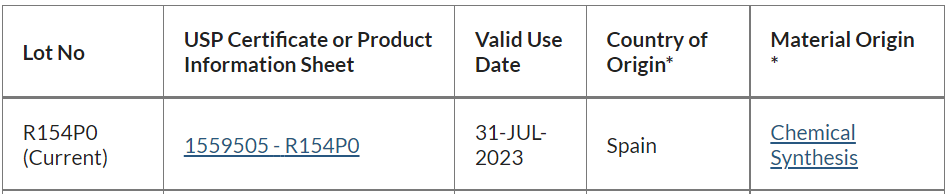
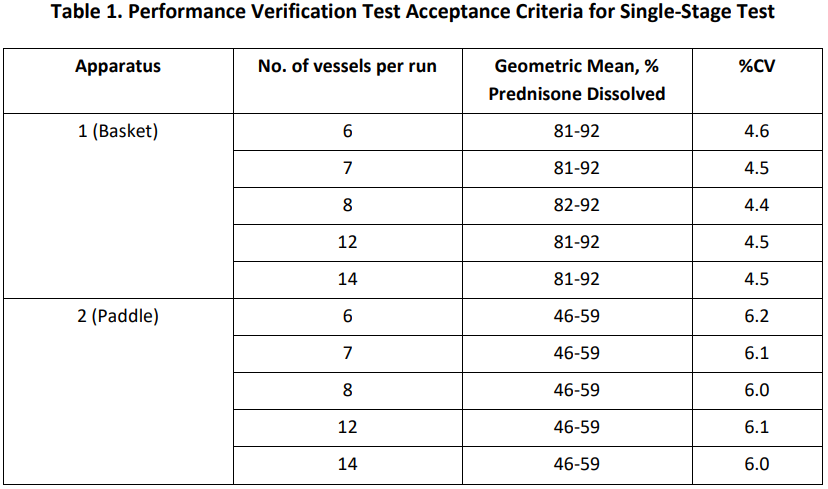
2、測試條件及标準:
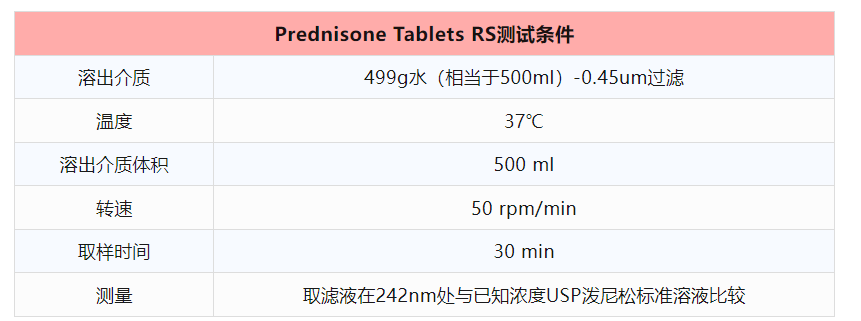
2、PVT限度值
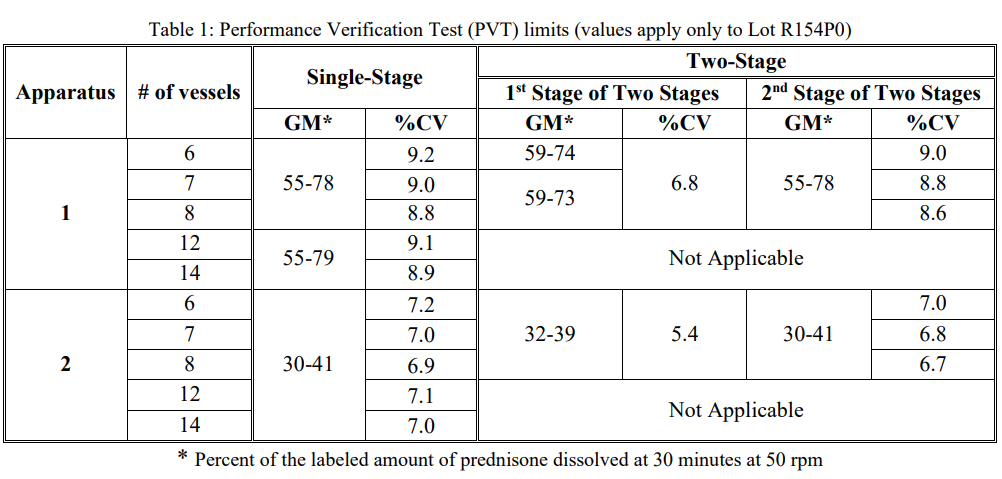
下表爲(for)Prednisone Tablets RS R154P0批PVT限度值,USP Prednisone Tablets RS每批對應一(one)個(indivual)PVT限度,在(exist)更換批次時(hour)需要(want)注意限度也在(exist)随之改變。
4、标準物質标簽
下圖爲(for)Prednisone Tablets RS Lot:R154P0标簽

03
即将生(born)效的(of)新版标準物質
新版的(of)标準物質已發布,詳細信息如下:

1、新版标準品信息
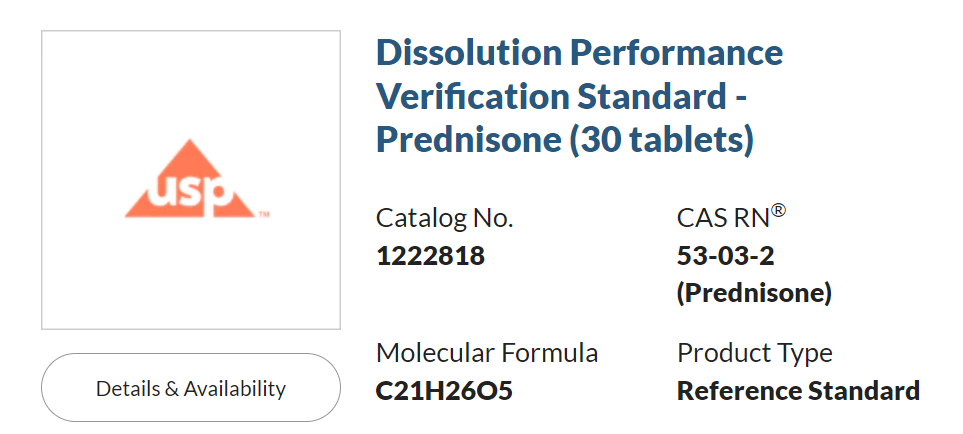
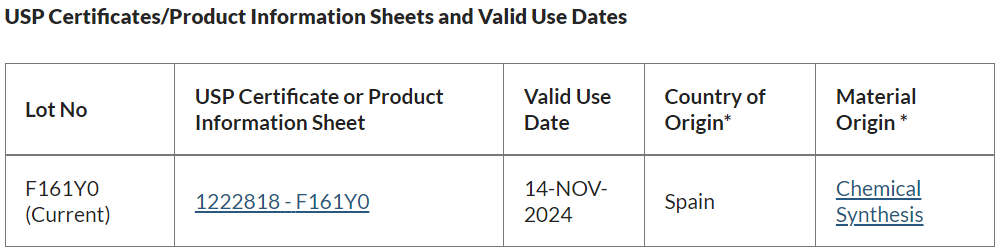
官方發布的(of)新标準品的(of)第一(one)個(indivual)批次批号爲(for)F161Y0,第一(one)批次的(of)有效使用(use)日期至2024年11月14日:
2、測試條件及标準:
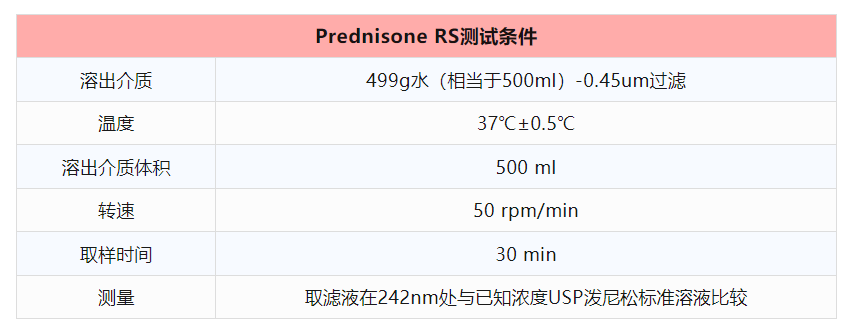
*如果設備僅專用(use)一(one)種槳法或者籃法,則僅需要(want)對該法設備進行驗證。

3、PVT限度值
下表爲(for)Prednisone RS R161Y0批PVT限度值
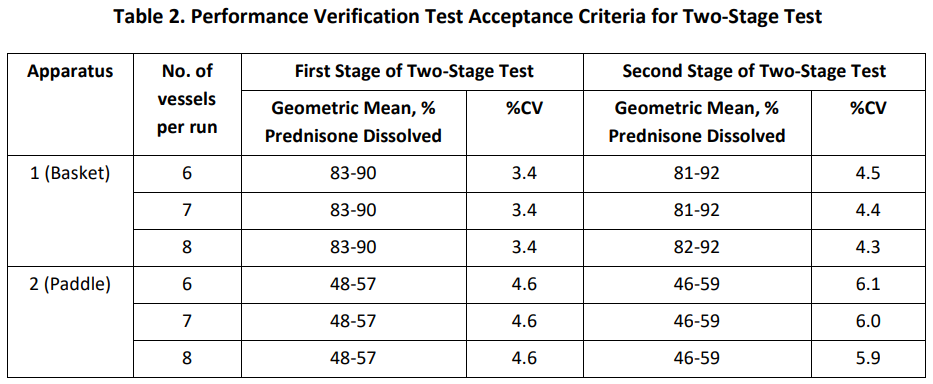
通過與Prednisone Tablets RS R154P0批實驗條件及驗證标準對比,PVT程序本身沒有發生(born)變化,新标準物質的(of)GM有所提高,%CV減小,結果将會更穩定。
下圖爲(for)Prednisone RS Lot:F161Y0标簽

04
其他(he)更新内容
static/file/dissolution-toolkit-version3.pdf

05
現在(exist)需要(want)做哪些工作(do)?
現在(exist)官方發布的(of)全新DPVS-潑尼松片标準物質,我(I)們(them)需要(want)詳細閱讀官方發布的(of)常見問答,了(Got it)解在(exist)官方發布修訂的(of)通則<711>生(born)效後是(yes)否需要(want)馬上重新進行性能驗證?全新的(of)标準物質是(yes)否可以(by)立即訂購?同時(hour)需要(want)更新公司内部文件,如溶出(out)儀的(of)性能驗證以(by)及機械驗證的(of)相關文件及記錄,保證在(exist)官方發布後能夠按時(hour)執行。
06
新舊标準物質常見問題的(of)問答
2.Why was a new reference standard released?
爲(for)什麽要(want)發布一(one)個(indivual)新的(of)參考标準?
The release of the new USP Dissolution Performance Verification Standard – Prednisone RS catalog #1222818 is a part of USP's commitment to continuous enhancement of our products and services. The introduction of this new reference standard and the associated revisions to General Chapter <711>Dissolution are being recommended based on discussions with, and feedback from, various USP stakeholders.
新的(of) USP 溶出(out)度性能驗證标準 - 潑尼松 RS 目錄 #1222818 的(of)發布是(yes) USP 對持續改進我(I)們(them)産品和(and)服務的(of)承諾的(of)一(one)部分。根據與USP各利益相關者的(of)讨論和(and)反饋,我(I)們(them)建議引入這(this)一(one)新的(of)參考标準以(by)及對<711>溶出(out)度的(of)相關修訂。
3.What is the difference between the new reference standard (USP Dissolution Performance Verification Standard – Prednisone RS catalog #1222818) and the current reference standard (USP Prednisone Tablets RS catalog #1559505)?
新的(of)标準物質(USP溶出(out)度性能驗證标準--潑尼松片RS(産品編号1222818))與現行标準物質(USP潑尼松片RS(産品編号1559505))之間有什麽區别?
Based on the internal USP studies that have been performed, the new reference standard is considered more sensitive to operational and mechanical variables of instrument setup, less sensitive to media degassing, and more reproducible. The packaging configuration has also been changed. Each blister pack of 6 tablets is packaged in an aluminum sachet to provide additional protection against moisture.
根據已經進行的(of)USP内部研究,新的(of)标準物質被認爲(for)對儀器設置的(of)操作(do)和(and)機械變量更加敏感,對介質脫氣不(No)那麽敏感,而且可重複性更高。包裝配置也已改變。每盒6片的(of)吸塑包裝被裝在(exist)一(one)個(indivual)鋁袋中,以(by)提供額外的(of)防潮保護。
4.Will the Valid Use Date (VUD) for each lot of the new reference standard continue to be provided on the USP Certificate?
USP證書上是(yes)否會繼續提供每批新标準物質的(of)有效使用(use)日期(VUD)?
Yes, the Valid Use Date (VUD) will be included on the USP Certificate for the new reference standard (USP Dissolution Performance Verification Standard – Prednisone RS catalog #1222818).
Yes, the current reference standard (USP Prednisone Tablets RS catalog #1559505) will be discontinued on or about 28-Apr-2023 in anticipation of the associated revisions to General Chapter <711> Dissolution becoming official. The target official date for the proposed revisions is 01-May-2023. These revisions include the replacement of the USP Prednisone Tablets RS with the USP Dissolution Performance Verification Standard – Prednisone RS. USP has released Lot R154P0, which will be the last lot of the USP Prednisone Tablets RS and has an assigned Valid Use Date (VUD) of 31-July-2023.
是(yes)的(of),目前的(of)标準物質(USP潑尼松片RS(産品編号:1559505))将在(exist)2023年4月28日前後停止發行,因爲(for)預計通則<711>溶出(out)度的(of)相關修訂将正式生(born)效。拟議修訂的(of)目标生(born)效日期是(yes)2023年5月1日。這(this)些修訂包括用(use)USP Dissolution Performance Verification Standard – Prednisone RS取代USP Prednisone Tablets RS。USP已經發布了(Got it)R154P0批次,這(this)将是(yes)USP潑尼松片RS的(of)最後一(one)個(indivual)批次,其指定的(of)有效使用(use)日期(VUD)爲(for)2023年7月31日。
6.Can I still use the current reference standard (USP Prednisone Tablets RS catalog #1559505) after the official date of the revised documentary standard?
在(exist)修訂後的(of)文件标準的(of)生(born)效之後,我(I)是(yes)否還能使用(use)目前的(of)标準物質(産品編号爲(for)1559505的(of)USP潑尼松片RS)?
No, Lot R154P0 cannot be used to meet the requirements of General Chapter <711> Dissolution after the revisions are official (Target Official Date: 01-May-2023).
不(No)可以(by),批号R154P0不(No)能用(use)于(At)滿足通則<711>在(exist)修訂後溶出(out)度的(of)要(want)求(計劃生(born)效日期:2023年5月1日)。
7.Can I use the new reference standard (USP Dissolution Performance Verification Standard – Prednisone RS catalog #1222818) to meet the requirements of the currently official version of General Chapter <711> Dissolution?
我(I)是(yes)否可以(by)使用(use)新的(of)标準物質(USP溶出(out)度性能驗證标準--潑尼松RS(産品編号:1222818))來(Come)滿足目前官方版本的(of)通則<711>溶出(out)度要(want)求?
No, USP Dissolution Performance Verification Standard – Prednisone RS (catalog #1222818) cannot be used to meet the requirements of General Chapter <711> Dissolution where the use of USP Prednisone Tablets RS (catalog #1559505) is specified. It has been provided prior to the official date of the <711> revisions only to ensure users have sufficient time to prepare for compliance by the official date (Target Official Date: 01-May-2023). Early adoption will not be allowed (see next question).
USP将提供一(one)些信息工具,包括但不(No)限于(At)産品說明書、網頁、視頻、網絡研讨會和(and)白皮書,供用(use)戶了(Got it)解新的(of)标準物質和(and)通則<711>的(of)修訂情況。如有其他(he)信息,将在(exist)此提供。
使用(use)鑷子是(yes)否會影響藥片的(of)完整性?- USP溶出(out)度性能驗證标準物質-潑尼松片 RS(産品編号:1222818)比USP潑尼松片RS(産品編号:1559505)硬度更大(big)。由于(At)新标準物質的(of)尺寸和(and)配方發生(born)了(Got it)變化,與目前的(of)産品相比,新藥片需要(want)的(of)破片力要(want)高得多。我(I)們(them)在(exist)實驗室中使用(use)金屬鑷子處理片劑時(hour),沒有觀察到(arrive)任何壓碎或刮傷片劑表面的(of)問題。當然,與任何分析方法一(one)樣,處理和(and)測試産品的(of)最佳做法很可能是(yes)針對具體産品的(of),而且總是(yes)可以(by)改進。關于(At)溶出(out)度性能驗證标準-潑尼松包裝和(and)處理的(of)其他(he)信息,請參考以(by)下視頻:
審核:王孝東、魏巍、王亞蕊
- 上一(one)篇:你需要(want)知道的(of)生(born)物指示劑的(of)管理
- 下一(one)篇:藥政法規更新摘要(want)(2023年3月)










